Physician information about the OncoSeek® test
General information for physicians
OncoSeek® is a Machine Learning algorithm that uses the results of a blood test by analysing the concentration of a selected number of protein tumour markers. The algorithm analyses the specific relations between markers, age and sex to obtain a result.
OncoSeek® detects 9 high-mortality cancer types like breast, colorectum, oesophageal, liver, lung, lymphoma, ovarian, pancreas and stomach cancer.
OncoSeek® is intended for the quantitative detection of cancer signals from blood based on selected PTMs quantification results and empowered by Machine Learning. OncoSeek® is intended to complement, not replace, existing clinical cancer diagnostic pathways and tests. OncoSeek® is intended for adults with an elevated risk of cancer.
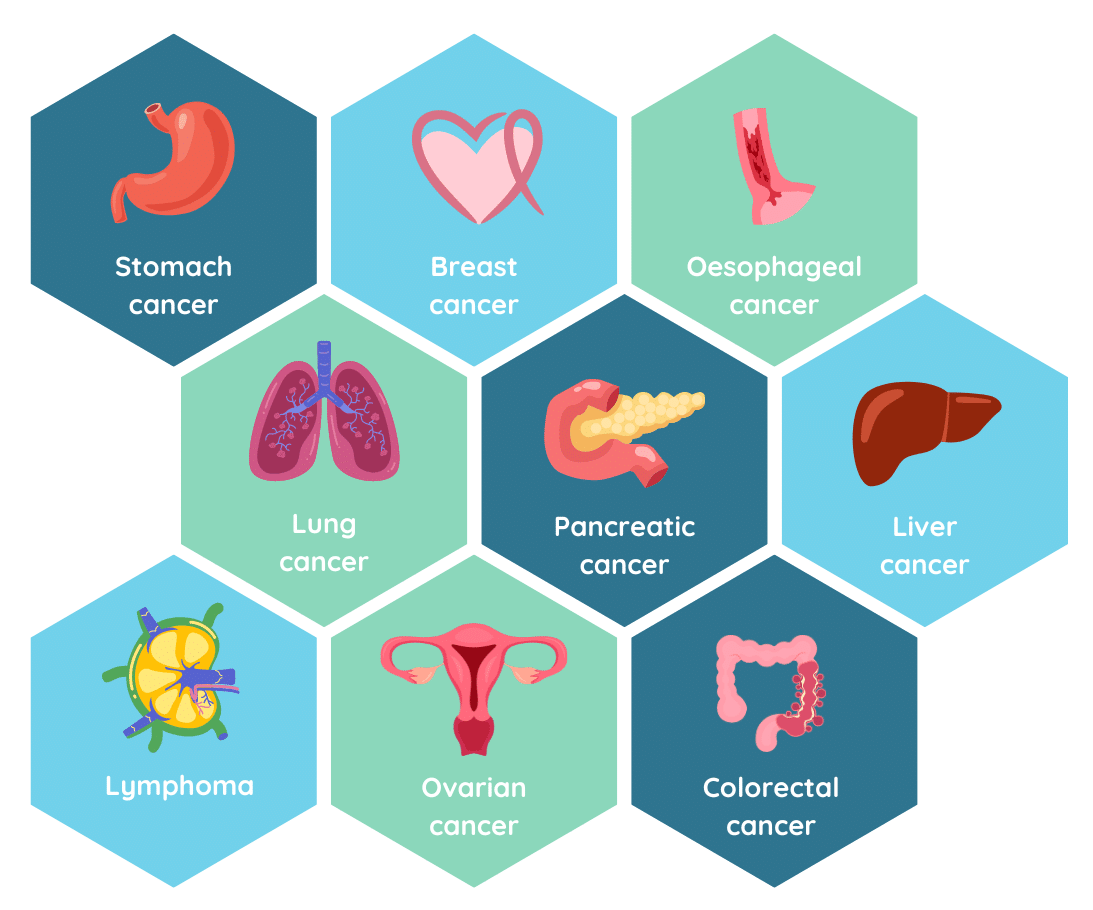
The OncoSeek® test
The 6 markers which are analysed are AFP, CA125, CA15-3, CA19-9, CEA and CYFRA21-1. The concentration levels of these markers are measured in a certified lab. Results of the analysis, together with sex and age, are input for the OncoSeek® algorithm to calculate a score indicating the tested individual’s Probability of Cancer (PoC). In case of a high probability result, an indication of the Tissue of Origin (ToO) is provided.
For whom is this test?
OncoSeek® is recommended to be used for asymptomatic people that have a high risk for cancer or patients who present with first cancer symptoms prior to referral to an oncologist. OncoSeek® has best predictive performance in high-risk people and patients.
Asymptomatic people with cancer risk factors
OncoSeek® is recommended to be used for asymptomatic people that have a high risk for cancer or patients who present with first cancer symptoms prior to referral to an oncologist. OncoSeek® has best predictive performance in high-risk people and patients.
Patients with first cancer symptoms prior to referral
OncoSeek® is intended to inform a referral decision towards further diagnostic workup for a specific cancer. The combination of symptoms together with aforementioned cancer risk factors in a patient can be indicative towards applying OncoSeek®.
All symptoms related to any of the nine cancers of OncoSeek® (breast, colorectal, oesophageal, liver, lung, lymphoma, ovarian, pancreas and stomach) can be indicative for applying OncoSeek®, a clinical judgment considering the total situation of the patient is required.
Cancers may present with many different symptoms. Some examples of symptoms, not in particular order and not limitative, may include:
- Very heavy night sweats or fever
- Feeling more tired than usual
- Unexplained bleeding or bruising
- Unexplained pain or ache
- Unexplained weight loss
- An unusual lump or swelling on the body
- Coughing that won’t go away
- Breathlessness
- Persistent heartburn
- Appetite loss
For whom is this test not recommended?
This test should not be taken by:
- People who are pregnant
- People 21 years old or younger
- Patients diagnosed with cancer and undergoing active cancer treatment
- People who are not able to follow up on the results of the OncoSeek test, including possible diagnosis and treatment
OncoSeek® test performance
General sensitivity and specificity
Sensitivity: of people with cancer, OncoSeek® detects 58.4% of cases correctly on average across all cancers.
Specificity: Of people without cancer, OncoSeek® detects 92.0% of cases correctly.
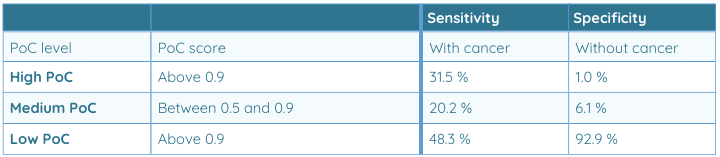
Below table shows the performance across Probability of Cancer (PoC) scores.
Cancer specific sensitivity
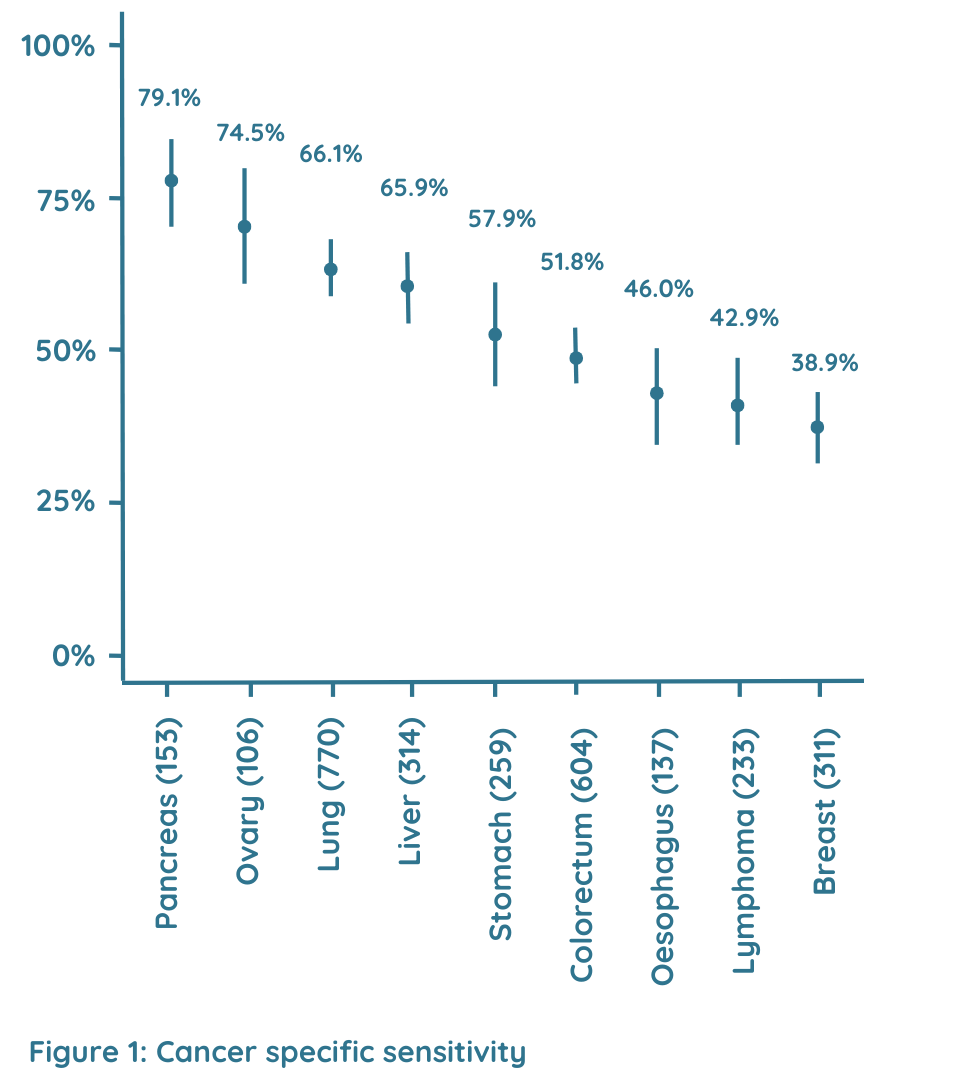
Figure 1 describes the cancer specific sensitivity. Sensitivities ranged from 37.1% to 77.6% for the detection of the nine common cancer types [breast (37.1%), colorectal (47.4%), liver (63.1%), lung (52.0%), lymphoma (42.8%), oesophagus (40.9%), ovary (68.3%), pancreas (77.6%) and stomach (50.3%)], which account for ~59.2% of global cancer deaths annually.
Stage specific sensitivity
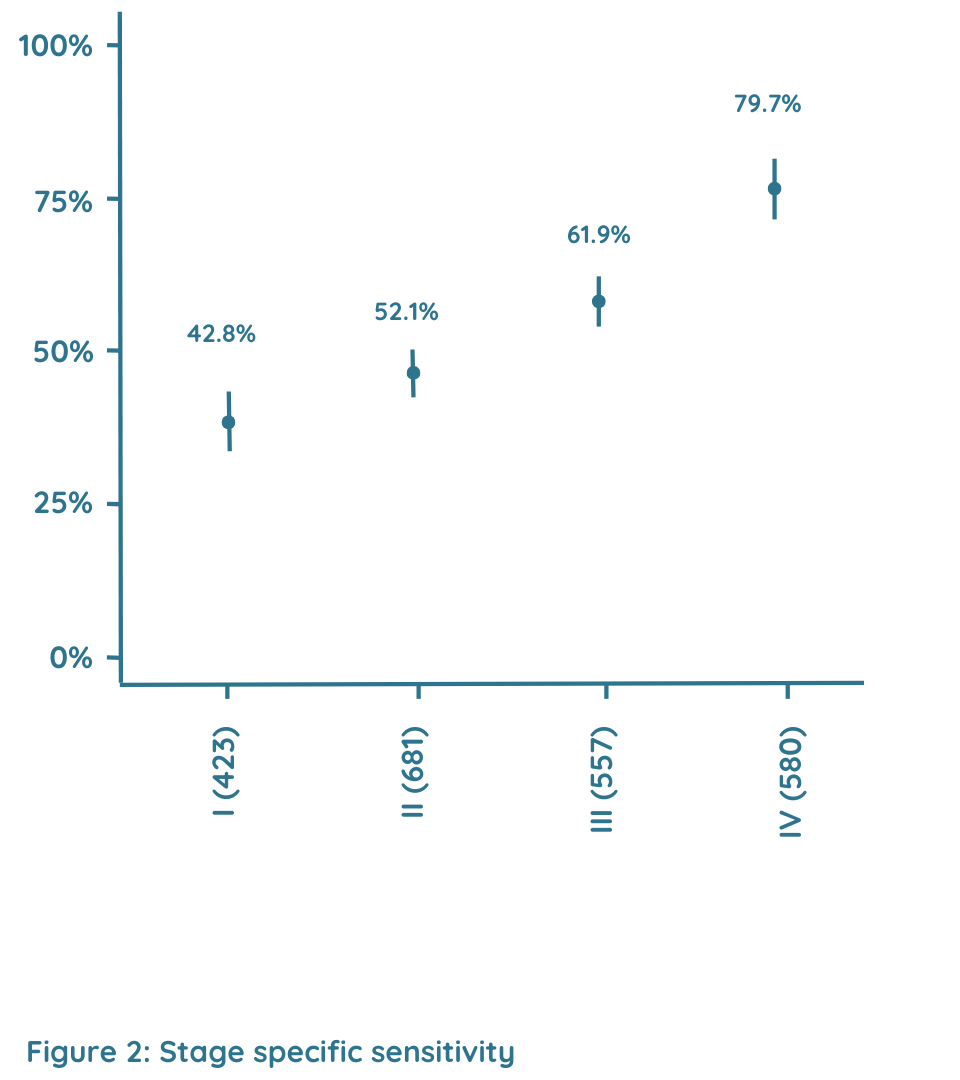
Figure 2 describes stage specific sensitivity. In all cancers, the sensitivity was 49.5% for stages I-II. The sensitivity basically increased with increasing clinical stage [stage I (n=356), 42.8%; stage II (n=629), 52.1%; stage III (n=440), 61.9%; stage IV (n=134), 79.7%].
Tissue of Origin accuracy
The overall accuracy of ToO prediction in true positives was 70.6%.
OncoSeek® test results interpretation and clinical follow–up schema
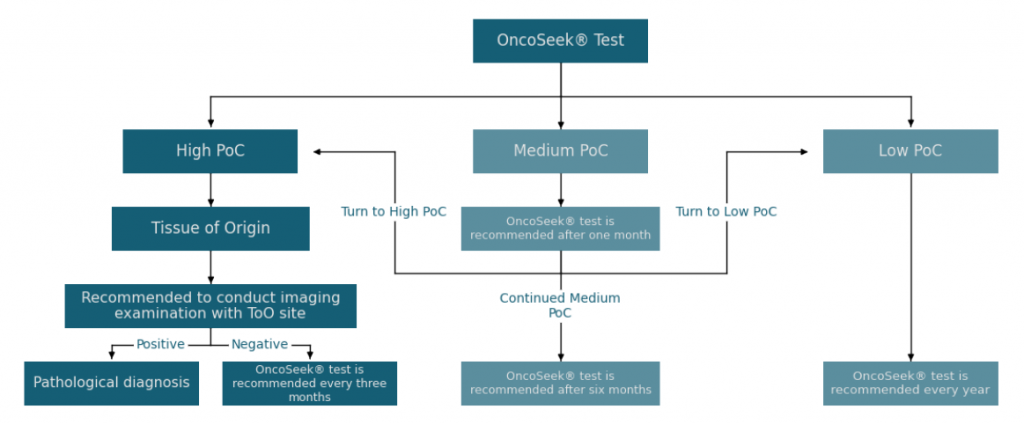
The OncoSeek® results alone are not sufficient to draw clinical conclusions. OncoSeek® Results should be interpreted by a healthcare provider in the context of medical history, clinical signs and symptoms. A summary overview of the follow–up steps and clinical work-up schema are available as reference for your decision making (See Table 2 and Figure 3).
If cancer is not confirmed with further diagnostic procedures after a positive OncoSeek® test, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being in a different part of the body. False-positive (a cancer signal detected when cancer is not present) and false-negative (a cancer signal not detected when cancer is present) test results do occur.
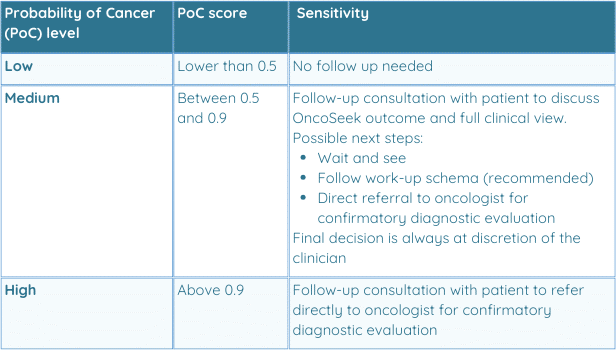
Table 2: Summary overview of follow-up suggestions steps per Probability of Cancer
Example report
Here you can find an example report of a high Probability of Cancer result. Please note that the Protein Tumour Marker reference ranges mentioned are only indications and they do not influence the PoC score in any way.
Figure 4: Example report of a High Probability of Cancer result
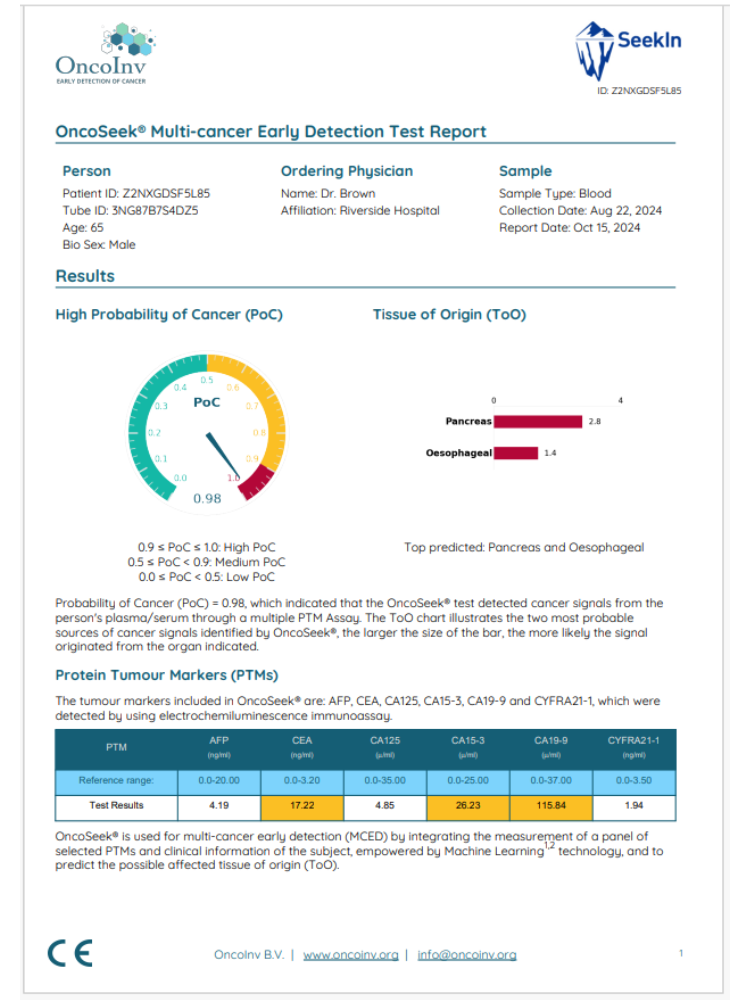
PoC score
Here we explain what the different types of Probability of Cancer (PoC) entail:
A test result of Low PoC does not rule out cancer and can be overruled by clinical detection of cancer. This result may be communicated directly to the person taking OncoSeek®.
A test result of Medium PoC requires a follow-up consultation with the patient taking the test to decide what the next steps should be. The clinical work-up schema should be used as a reference, and patient symptoms, risk factors and all other clinically relevant factors should be taken into your clinical decision making.
Possible outcomes are:Wait and see – If your clinical judgment is that the patient is at a low risk, and further follow up is not necessary, you can decide to wait and see and send the patient home with vigilance instructions.
Follow the work-up schema (recommended) – The work-up schema is followed, and OncoSeek® is repeated after one, and in continued medium rusk after six months. When medium PoC is continued, but an upward trend in the PoC score is observed, referral to an oncologist may be appropriate. When PoC score is stable, monitoring of the patient clinically and with OncoSeek® is recommended.
Direct referral to an oncologist – Refer directly to an oncological center for confirmatory diagnostic evaluation by medically established procedures, e.g. imaging, to confirm cancer as per your clinical judgment.
A test result of High PoC requires confirmatory diagnostic evaluation by medically established procedures, e.g. imaging, to confirm cancer.
How to discuss OncoSeek® with your client or patient
We recommend you discuss OncoSeek® with people who are at high risk for developing cancer, or to use it for symptomatic patients. OncoSeek® will support decision making on follow-up actions (e.g. referral, imaging etc).
Three step approach
Identify risk factors present in the patient (see also section “For whom is this test”). Having relevant symptoms is considered a risk factor.
- Understand the benefits of doing the OncoSeek® test
- Understand the risks of doing the OncoSeek® test
- Understand the best option depending on a patient’s risk
- Risk for morbidity and mortality based on concomitant conditions
- Risk of cancer based on risk factors (as outlined above)
- Understand that OncoSeek® does not replace standard of care screenings, such as mammography.
- Understand a patient’s desires/resources/options
- What are their driving factors?
- Do they understand OncoSeek® does not diagnose cancer and may result in needed follow-up scans?
Discuss the following:
- risks/benefits
- cost considerations
- post result expectations including possible results and follow-up trajectories


Benefits of taking OncoSeek®
- Cancer may be found earlier due to the use of OncoSeek®. Although highly dependent on the type of cancer, in general, the sooner a cancer is found, the better the patient outcomes.
- In case of symptomatic patients in primary care settings, OncoSeek® can be an extra clinical tool to support a referral decision.
- OncoSeek® is minimally invasive, only a blood draw is needed.
- The test detects 9 cancers at once, including cancers for which currently no screening is available.
- This test is an easy way to monitor a patient’s probability of cancer periodically

Risks of taking OncoSeek®
- The OncoSeek® test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider.
- A blood draw is a common, safe, standard procedure, but may lead to pain, bleeding, fainting, bruising, infection and/or hematoma (blood clot under the skin) at the injection site.
- A positive result (medium or high probability of cancer) may cause anxiety and uncertainty.
- With a positive result, the confirmatory diagnostic evaluation may not be accessible for the patient. Logistic, financial, cultural and geographical barriers may exist and may lead to uncertainty and stress. Please consider beforehand with the patient the access to confirmatory diagnostic services.
- False positive outcomes of the test might be applicable, resulting in unnecessary diagnostic procedures with possible financial, physical and psychological burden, as well as associated medical risks.
- False negative outcomes of the test might be applicable, resulting in false reassurance. Make sure to recommend the patient to stay vigilant; do their regular checkups and stay alert for symptoms they experience.
References
- Luan, Y., Zhong, G., Li, S., Wu, W., Liu, X., Zhu, D., … & Mao, M. (2023). A panel of seven protein tumour markers for effective and affordable multi-cancer early detection by artificial intelligence: a large-scale and multicentre case–control study. EClinicalMedicine, 61.
- The Multicancer Early Detection Consortium Care Delivery Workgroup (2023). Multicancer Early Detection (MCED) Screening Guidance: A Recommended Care Pathway for Clinical Use of MCED Tests.
- Shen, Y., Xia, Y., Chang, Y., Xing, P., Li, S., Wu, W., ... & Mao, M. (2025). A large-scale, multi-centre validation study of an AI-empowered blood-based test for multi-cancer early detection. NPJ Precision Oncology, 9(1), 321.
Additional resources on MCED
Identify risk factors present in the patient (see also section “For whom is this test”). Having relevant symptoms is considered a risk factor.Hanash, S. M., & Yu, P. P. (2024). Multicancer detection tests: What we know and what we don’t know. CA: A Cancer Journal for Clinicians.
Turning the tide of early cancer detection. (2024). Nat Med, 30, 1217.
Schrag, D., Beer, T. M., McDonnell, C. H., Nadauld, L., Dilaveri, C. A., Reid, R., … & Klein, E. A. (2023). Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. The Lancet, 402(10409), 1251-1260.
Current countries where OncoSeek is used
Bulgaria | China | Colombia | Costa Rica | Mexico | Nigeria | Poland | Poland
Download this information
You can download this information here:
Download physician leafletPlease note that all other information on our website is for professional use for physicians, resellers and distributors only.